Recently in anatomy class we took our final we did diagrams and test questions and multiple choice. I think I did fairly well except the fact that I forgot all the muscles and I forgot my notebook at home. In AP Chemistry class we tie dyed for our final. It was fun. What I'm doing next is graduating. I can't wait. I'm still thinking about where I want to go, I have a good scholarship here but I also have a good scholarship to University of Dallas, and they want me to play soccer. What I'm going to do in the future is learn from my mistakes and AP Chemistry quit procrastinating and when it gets tough don't quit.
Friday, May 8, 2015
Friday, April 24, 2015
3 Qs
ecently in anatomy class we learned about the urinary system. we learned about the kidneys and filtration and secretion. we also got to make urine which is pretty cool. We used stimulated blood type A & B and put it in dialysis tubing, then we tied up the dialysis tubing from end to end and place it in a cup of DI water. We also recently had a take home test for the urinary system. in AP chemistry we finished our equilibrium unit and are now reviewing for the AP test. we took a practice AP test and are now looking at the areas we need to hit up on. I hope to do well on the AP test.
Friday, April 10, 2015
3 Questions
Recently I have learned about pH and Poh in AP chemistry and that's learned about the respiratory system in anatomy. I learned about how co2 is diffuse through the body and how o2 is diffused to the body as well. we have a lab coming up determining an unknown acid concentration by titration which we will be able to determine by its Poh and pH levels. I hope to ace my respiratory system test in anatomy. I also hope to go in and see Ms. Gardner whenever I am unsure about a concept in chemistry.
Thursday, April 9, 2015
Colorimetry in pH levels
The difference in pH between an acid and a base is the measurements on a pH scale. An acid is below 7, and a base is above 7. When they cancel each other out its a 7, which is neutral. An acid is a proton donor, while a base is a proton reciever. pH= -log[H+] and pOH=-log[OH-]. The relationship between pH and pOH is that they both equal 14. The product of the concentrations of hydrogen ions and hydroxide ions equals Kw. pH indicators are substances, usually a weak acid or base, that changes color depending on the pH of the solution it is mixed with. Indicators don't change color sharply at one particular pH level. They change over a narrow range of pH. And this color change interval is different for each indicator. The difference that we observe between UI and other indicators is the composition of the indicator whether it is a base or acid and the color. I would choose different indicators. Although a pH of 5 and pH of 9 might seem close, they are very different on the pH scale, so it would be better to use different indicators. Indicators react with the hydrogen or hydroxide ions in the different substances. They change its formula and color to qualitatively show a pH or pOH scale. Most indicators only show a certain range of colors like Methyl orange or meta cresol, because of its limited pH indicators. The UI has the ability to show all the colors on the pH scale whereas Methyl orange could only show colors ranging from red to yellow and bromothymol blue can only show colors ranging from yellow to blue.
Monday, March 30, 2015
Equilibrium Explore Lab
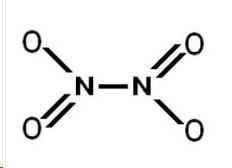 |
| Dinitrogen Tentroxide |
 |
| Nitrogen Dioxide |
In each test tube was the Nitrogen Dioxide (NO2) and the Dinitrogen Tentroxide (N2O4). The No2 is favored by the heat and produces a orange yellowish gas. The N2O4 is colorless and is favored by the cold. The formation from 2NO2 ----> N2O4 is exothermic. The reverse reaction is endothermic because there is an increase in energy needed to break bonds. The formation of N2O4 releases more energy and the energy reversed requires a lot more due to the creation of 2 NO2. The rate of the reaction in both reactions is equal. Its the same in the reverse reaction as it is in the forward reaction. The change in color provides the evidence that one chemical was more favored in the other. This color change indicates a change in concentration, causing a change in energy, causing a different change in equilibrium. Because the color change the equilibrium changes. Equilibrium Is established in different temperatures. It wants to adjust and find a new equilibrium when the color changes. The reactions are happening continually and simultaneously. All three different points of the reaction were at equilibrium, but they were all at different points because of the change in temperature. The equation is ;
2NO2------><------- N2O4
The enthalpy for the forward reaction is -58.8 KJ/ mol of N2O2, and the enthalpy of the reverse reaction will be +58.8 KJ/ mol of 2No2. In the LeChatelier's principle is used to manipulate the outcomes of reversible reactions, often to increase the yield of reactions.
Friday, March 27, 2015
3 questions
Recently I've learned about kinetics. I taking the test over kinetics in my AP Chemistry class. I hope to do well on that test. we just started a new unit on equilibrium. this will be our last chapter before reviewing for the AP chemistry test. we gotta work sheet over it to do during spring break, it's pretty easy. We just finished the cardiovascular system in anatomy and I got the highest score on the test. I think we're learning about the urinary system next. I hope in this next chapter for chemistry that I will go in during lunch. whenever I need help or are you stuck on something instead of just hiding my problems go in and ask for help.
Friday, March 13, 2015
3 Questions
Recently I've learned about the blood in anatomy and kinetics in ap chemistry. I've I've learned about blood types and the cardiac output, also the anatomy of the heart. In Ap chemistry I learned about reaction mechanisms and half lives and catalysts. I'm still struggling with some of the mathematical concepts in this class and I hope to come in during lunch.
Monday, March 2, 2015
8.1 Reactions & Rates PhET
Describe what affects the potential energy of the particles and how that relates to the energy graph.
Describe how the reaction coordinate can be used too predict whether a reaction will proceed slowly, quickly, or not at all.
The reaction coordinate can use to predict whether a reaction will proceed slowly quickly or not at all is by its total energy. If the total energy is high the reaction will occur very quickly, if the reaction is slow then the total energy will be very low. And this will be determined and shown on the reaction coordinate graph.


Friday, February 27, 2015
3 qs
Recently in anatomy class we learned about the endocrine system. We took a test over it last week to test our knowledge on endocrine and reproductive systems. We learned about different hormones and the anatomy of the reproductive system and functions in both male and female. In chemistry class we learned about solids in Lewis structures and when you're more in depth on intermolecular forces and intramolecular forces. I take my test Monday. I hope to get a good grade on this test I am going to study and watch videos in the cover the notes so I know I understand the learning objectives. I am going to go in for help more often more than once a week like I normally do and start to retake my quizzes instead of procrastinating.
Friday, February 13, 2015
3 questions
Recently in my AP class we have learned about electronegativity and Lewis structures and covalent bonds. We did a lab today where we built structures out of atoms and compare them to our lewis structures that we drew then we figured out the bond shape and angle of each compound. In anatomy we recently learned about the endocrine system and about the different hormones and glands and where they are located and anatomical structure and physiology. What I'm about to do next is going during lunch to practice my stoich and equation solving skills. Hopefully I'll just be able to let it clicked in my head and quit making mistakes on my test in the free response.
Tuesday, February 10, 2015
Pepper and Soap Explore
What are the different intermolecular forces?
There are four types of intermolecular forces there is ionic forces, dipole forces, hydrogen bonding, and induced dipole forces. Ionic forces hold ions together in ionic solids and are electrostatic forces. Opposite charges attract each other they are the strongest intermolecular force. Dipole forces are polar covalent molecules and are sometimes described as dipoles meaning that the molecule has two poles. One end of the pool has a partial positive charge while the other end has a partial negative charge. They will orient themselves so that the opposite charges attract principle operate effectively. Hydrogen bonding is the attractive force between the hydrogen attached to an electronegative atom of one molecule and the end electronegative atom of the different molecule, usually its oxygen nitrogen or fluorine. The hydrogen is partially positive and attracted to the partially negative charge on the oxygen or nitrogen. Induced dipole forces are forces between essentially nonpolar molecules which is the weakest of all intermolecular forces these temporary dipoles attract or repel the electron clouds of nearby nonpolar molecules.
What intermolecular force does water experience?
Water experiences hydrogen bonding. The hydrogen has a very low electronegativity resulting in the oxygen atoms having greater affinity to the covalent shared electrons this makes the molecule extremely polar so the molecules stick together more.
What intermolecular force does soap seem to have?
Soap is different from water. It is nonpolar because water is polar this was observed when the pepper spread once the soap was dropped in the water. Some type of component that this soap had is nonpolar which reacted with the water which called the repeling of the pepper. After a little while the soap dissolved in spread into the water . Does soap acts as a phospholipid bilayer.
What happened when the soap was added to the pepper water? Why?
When the soap was added to the pepper water the pepper spread to the outside of the cup making it clear center of the water. This is because the soap and water has different intermolecular forces the water is polar and the soap is non polar. The pepper was used to show the repelling of the nonpolar vs polar.
Friday, January 30, 2015
Three ???
Recently we did a lab on the light in fd and C blue dye in Gatorade. We look to see the percent transmittance between different variations of the stock solution and water mixture. In anatomy we have been learning about neurons and the brain and central nervous system. We watched a movie about the grain. It was really cool we saw how panic can produce hormones that distrubs the stimulus of the brain and makes it not be able to focus on its normal functions. I am stuck struggling with the stoichiometry. I just get confused on where to put the units over what. Overall I enjoyed this unit I find it fairly easy to understand I'm actually understanding the electron configuration and wavelength and frequency is in emission spectrum and its very relieving that I can actually understand something in AP chemistry. I am planning to keep up my practices with the math equations and stoichiometry so I can better my struggles and perform better on tests and quizzes and overall get a higher grade in this class.
Monday, January 26, 2015
Shell Activity Blog
- What is Coulomb's law? Coulombs law is the basic idea of electricity. It looks at forces created between two charged objects. As distance increases the forces of electric fields decrease. When there are two charged particle, an electric tone is created. If there are longer charges the forces will be longer.
- Explain how distance and charge impact the energy to remove an electron. Why do both 'charge' and 'distance' need to be included in your explanation? Distance and charge are inversely related to each other. The closer the electron is to the nucleus (closer distance) the stronger the force. So the closer the electron is the more energy is needed to overcome the force of attraction between electron and proton. The further the electron, there is less energy needed.
- Explain why removing an electron is an endothermic process.and why it isn't an exothermic process. Removing an electron is an endothermic process because energy is absorbed to break the bond between electron and proton.
- How is the amount of energy needed to remove an electron related to how electrons are organized within an atom? The amount of energy needed to remove an electron is related to how electrons are organized within an atom.When the electron jumps to a lower suborbital it emits a photon, emitting light, This absorbs energy which csuses the emission of a photn t make the atom stable.
- How is the amount of energy needed to remove an electron related to, different than, or the same as, the energy to excite an electron? The energy needed to remove one or more electrons from a neutral atom to form a positively charged ion is a physical property that influences the chemical behavior of the atom. By definition, the first ionization energy of an element is the energy needed to remove the outermost, or highest energy, electron from a neutral atom.
Sunday, January 25, 2015
Light and Electons
- What is the relationship between wavelength, frequency and energy of light?
- How does light emission demonstrate electron transition?
- What is significant about the different colors of light observed in spectral lines?
- How can light be used to measure energy transitions of electrons?
Friday, January 16, 2015
Three Questions
Recently I have learned about the brain and its functions. I learned about the organize a shin of the central nervous system which includes the brain and spinal cord. The protection of the central nervous system . The disruption to neural functions, the metabolic processes, the layers of the cerebrum,and the sensory and motor areas of the cerebral cortex. In my AP Chemistry class I learned about light and electrons. Also a little brief review on frequency and wavelength. Recently we just completed a lab over the electrons and light in relate the white to the electron transition in the production of the light observed. Also I've recently completed a Play Doh brain model. I am planning to start to review my stoach and problem solving in my chemistry class so I can have a greater understanding of the whole concept and finally figure out what I need to learn and what I need to work on as the upcoming AP Chemistry test approaches, so I can get a good grade on that.
Thursday, January 15, 2015
Light and Electrons
The higher the frequency the shorter the wavelength and the lower the frequency the longer the wavelength. The longer the wavelength the lesser the energy . Wavelength and energy of light are indirect. Wavelength is also indirect with frequency. So frequency and energy of light are directly related. light emission demonstrates electron transition by spectral lines. To produce these spectral lines you need to have an electron. Each electron produces one spectral line. For example a single atom of hydrogen cannot produce all for hydrogen spectral lines simultaneously. This is because hydrogen only contains one electron. Hydrogen gas can emit more than one spectral lines simultaneously because this is due to the multiple atoms used in your hydrogen gas example because there are more electrons there can be different spectral lines. The significance about the different colors of light observed in spectral lines are the wavelength. Each wavelength has its own unique color range. Red wavelength range range is from 625 to 740 nanometers, oranges range is from 592 to 625 nanometers, yellows range from 565 to 590 centimeters Greens range from 522 to 565 nanometers, blues range from 440 to 520 nanometers, and violets range from 382 to 440. There are different types of hues or shades of each color and they all each emitt different wavelengths. Every color has different energy; violets have the highest energy and the Reds have the lowest energy. So do to violet highest energy they have the shortest wavelength and red has the longest wavelength because they have the lowest energy. During electron transition energy can either be released or absorbed. When energy is released they are returning to their lower energy levels as they get closer to the nucleus its "ground state." when energy is absorbed it goes to higher energy levels to do this it has to absorb enough energy to break the attraction between the nucleus and the electron. The spectral lines for atoms are unique, different wavelengths are emitted to different atoms. If an electron moves from energy level 5 to energy levels to this electron transition releases energy. The electron moves from a higher energy state to a lower energy state. In this electron transition light is released because the energy is absorbed. The longer the wavelength the lower the frequency the shorter the wavelength the higher the frequency wavelength and frequency are indirect. Light can be used to measure energy transitions of electrons by looking at the wavelength ranges. An example is hydrogen electron transition that involves light with a wavelength in the ultraviolet range which is it 10 to four hundred nanometers can be measured n= 6 to 1. A hydrogen electron transition that involves light with a wavelength in the infrared range which is a thousand 2106 nanometers can be directed as n equals 6 to n equals 5.
Friday, January 9, 2015
Three questions
Recently in my anatomy class we learned about muscles. And we took a test over the muscular system yesterday. In chemistry we are reviewing thermochemistry. I hope to not procrastibate in this next upcoming semester and finish my classes with all as.







