The reaction coordinate can be used to predict whether a reaction will proceed including how the potential energy of the system changes. This diagram shows the energy of the reactants and products and therefore delta E. The highest point on the diagram is the transition state. The species present at the transition state is called the activated complex. The energy gap between the reactants and the activated complex is the activation energy barrier. activation energy in other words has a minimum amount of energy required for reaction. For example just as a ball cannot get over a hill if it does not roll up the hill with enough energy a reaction cannot occur unless the molecules possess efficient energy to get over the activation energy barrier. Reaction coordinate diagrams are helpful to visualize energy changes throughout a procession processed on a reaction coordinate diagram like this for reimbursement of molecules.
Describe what affects the potential energy of the particles and how that relates to the energy graph.
Describe what affects the potential energy of the particles and how that relates to the energy graph.
The factors that affect an object's potential energy are its height relative to some reference point its mass and the strength of the gravitational field.
Describe how the reaction coordinate can be used too predict whether a reaction will proceed slowly, quickly, or not at all.
The reaction coordinate can use to predict whether a reaction will proceed slowly quickly or not at all is by its total energy. If the total energy is high the reaction will occur very quickly, if the reaction is slow then the total energy will be very low. And this will be determined and shown on the reaction coordinate graph.
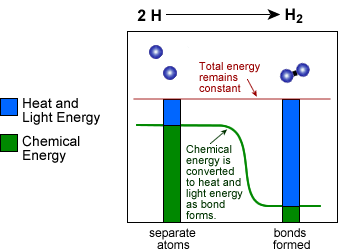


Describe how the reaction coordinate can be used too predict whether a reaction will proceed slowly, quickly, or not at all.
The reaction coordinate can use to predict whether a reaction will proceed slowly quickly or not at all is by its total energy. If the total energy is high the reaction will occur very quickly, if the reaction is slow then the total energy will be very low. And this will be determined and shown on the reaction coordinate graph.



No comments:
Post a Comment