In each test tube was the Nitrogen Dioxide (NO2) and the Dinitrogen Tentroxide (N2O4). The No2 is favored by the heat and produces a orange yellowish gas. The N2O4 is colorless and is favored by the cold. The formation from 2NO2 ----> N2O4 is exothermic. The reverse reaction is endothermic because there is an increase in energy needed to break bonds. The formation of N2O4 releases more energy and the energy reversed requires a lot more due to the creation of 2 NO2. The rate of the reaction in both reactions is equal. Its the same in the reverse reaction as it is in the forward reaction. The change in color provides the evidence that one chemical was more favored in the other. This color change indicates a change in concentration, causing a change in energy, causing a different change in equilibrium. Because the color change the equilibrium changes. Equilibrium Is established in different temperatures. It wants to adjust and find a new equilibrium when the color changes. The reactions are happening continually and simultaneously. All three different points of the reaction were at equilibrium, but they were all at different points because of the change in temperature. The equation is ;
2NO2------><------- N2O4
The enthalpy for the forward reaction is -58.8 KJ/ mol of N2O2, and the enthalpy of the reverse reaction will be +58.8 KJ/ mol of 2No2. In the LeChatelier's principle is used to manipulate the outcomes of reversible reactions, often to increase the yield of reactions.
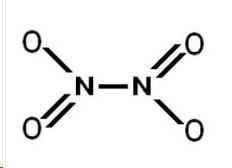 |
| Dinitrogen Tentroxide |
 |
| Nitrogen Dioxide |
In each test tube was the Nitrogen Dioxide (NO2) and the Dinitrogen Tentroxide (N2O4). The No2 is favored by the heat and produces a orange yellowish gas. The N2O4 is colorless and is favored by the cold. The formation from 2NO2 ----> N2O4 is exothermic. The reverse reaction is endothermic because there is an increase in energy needed to break bonds. The formation of N2O4 releases more energy and the energy reversed requires a lot more due to the creation of 2 NO2. The rate of the reaction in both reactions is equal. Its the same in the reverse reaction as it is in the forward reaction. The change in color provides the evidence that one chemical was more favored in the other. This color change indicates a change in concentration, causing a change in energy, causing a different change in equilibrium. Because the color change the equilibrium changes. Equilibrium Is established in different temperatures. It wants to adjust and find a new equilibrium when the color changes. The reactions are happening continually and simultaneously. All three different points of the reaction were at equilibrium, but they were all at different points because of the change in temperature. The equation is ;
2NO2------><------- N2O4
The enthalpy for the forward reaction is -58.8 KJ/ mol of N2O2, and the enthalpy of the reverse reaction will be +58.8 KJ/ mol of 2No2. In the LeChatelier's principle is used to manipulate the outcomes of reversible reactions, often to increase the yield of reactions.

No comments:
Post a Comment